Understanding Immune Privilege in Allogeneic Stem Cell Therapy
Why immune response may not be a barrier in veterinary regenerative medicine
Innate and adaptive immune system
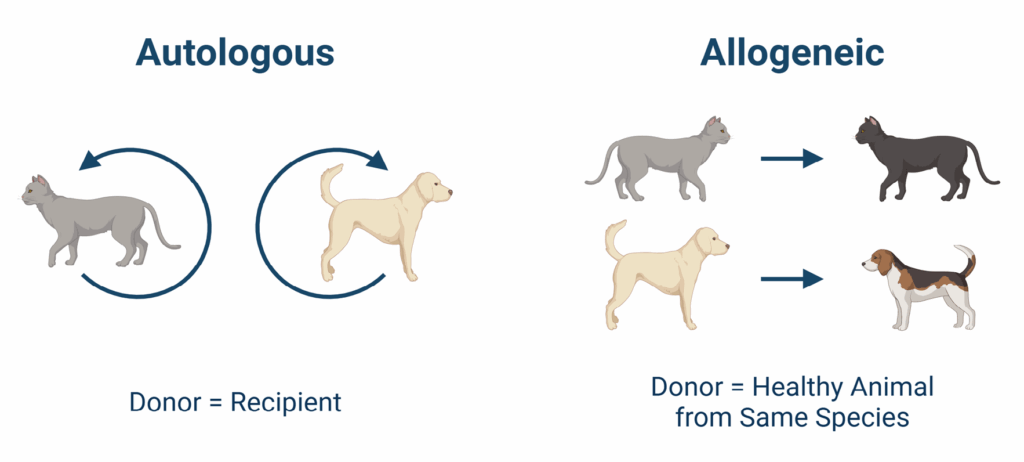
Feline Chronic Gingivostomatitis (FCGS), osteoarthritis, and other inflammatory diseases often leave pet parents and veterinarians searching for solutions that go beyond symptom management. One promising area of exploration? Allogeneic stem cell therapy—which uses cells donated by one animal to treat another.
A common question is: Won’t the immune system reject these cells?
This blog explores the concept of immune privilege in mesenchymal stem cells (MSCs) and what it could mean for the future of regenerative medicine in pets.
Note: Gallant’s investigational therapies are not approved for commercial use. All studies are conducted under FDA oversight.
What Does “Immune Privilege” Mean?
In immunology, “immune privilege” refers to cells or tissues that are less likely to trigger an immune response, even when introduced from a donor. This concept has been extensively studied in human medicine—and now, in veterinary medicine, it’s helping researchers understand how stem cells may work across individuals.
How Mesenchymal Stem Cells (MSCs) Avoid Rejection
MSCs are being evaluated for their immunomodulatory properties, meaning how they interact with the body’s own cells to balance the immune system.
Research suggests MSCs:
- Lack specific receptors called MHC receptors that are present on other cells and would normally trigger the body to recognize a cell as foreign and initiate an immune attack
- Suppress overactive immune responses
- Modulate inflammation
Because of these properties, MSCs may avoid detection or calm immune responses that would otherwise lead to rejection.
Why This Matters for Veterinary Care
If MSCs from healthy donors can be administered without triggering an immune reaction, it unlocks several advantages:
- Off-the-shelf availability
- No need for patient cell harvesting or culturing
- Consistent formulation, dosing, and delivery
These features could make regenerative therapies more accessible to general practice veterinarians—and more timely for the pets who need them.
Still Under Investigation
While early studies are promising, Gallant’s stem cell therapies remain investigational and are being rigorously evaluated under FDA-authorized clinical trials. These studies are essential to better understand how MSCs behave in real-world veterinary settings.
Want to Learn More?
We’re actively enrolling cats into studies evaluating investigational stem cell therapy for a variety of conditions.
Learn about our clinical trials, participation, and locations.
Disclaimer
Gallant’s investigational stem cell therapies are not commercially available. These veterinary products may be available through participation in a study at a qualified clinic under FDA-authorized protocols. This blog is intended for educational purposes only and does not constitute medical advice.