Stem Cell Therapy for Canine Atopic Dermatitis (CAD): Investigating a New Way to Help Dogs with Chronic Itchy Skin
Atopic dermatitis is one of the most common skin conditions in dogs. Gallant is studying how stem cell therapy may one day help address inflammation at its source.
Understanding Atopic Dermatitis
Atopic dermatitis is a chronic inflammatory skin condition that often starts in young to middle-aged dogs. Breeds such as Labrador Retrievers, Golden Retrievers, and West Highland White Terriers are particularly prone.
In dogs with atopic dermatitis, the immune system becomes unbalanced and overreacts to harmless things in the environment, like pollen or dust mites. This triggers certain immune cells, especially T helper 2 (Th2) cells, to release signaling molecules called cytokines. These cytokines cause inflammation, itchiness, and damage to the skin’s protective barrier. As the barrier weakens, more allergens and bacteria can enter the skin, which makes the immune reaction even stronger and creates a cycle of irritation and inflammation.

Common signs include:
- Persistent scratching or licking
- Red, irritated skin
- Hair loss from scratching
- Recurrent ear infections
- Thickened or darkened skin over time
These symptoms may wax and wane depending on allergen exposure and can worsen with secondary bacterial or yeast infections.
Why Current Treatments May Not Be Enough
Conventional therapies including steroids, cyclosporine, JAK inhibitors, and monoclonal antibodies can reduce inflammation and itching. However, they often target symptoms rather than the underlying immune imbalance that drives the disease.
As a result, some dogs experience incomplete relief or recurring flare-ups, leaving pet parents searching for longer-lasting options.
Investigating Stem Cell Therapy for Atopic Dermatitis
Stem cell therapy, a form of regenerative medicine, is being studied in atopic dermatitis for its potential to modulate the immune system and reduce inflammation. Gallant is evaluating mesenchymal stromal (stem) cells (MSCs) derived from uterine tissue, a renewable and ethically sourced cell type collected during routine spay procedures. These uterine-derived MSCs (UMSCs) are being investigated for their unique immunomodulatory properties, including their ability to regulate inflammatory signals in the body.
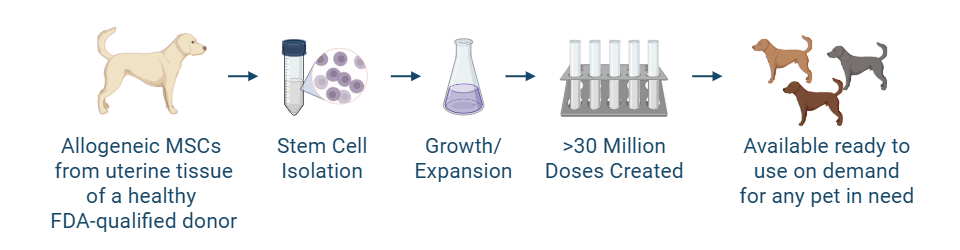
MSCs have been explored for atopic dermatitis in several small studies in dogs. In a Gallant pilot study, intravenous administration of allogeneic (donor-derived) UMSCs was associated with improved itchiness and skin lesions in dogs with atopic dermatitis. While these early findings are encouraging, additional studies are underway to demonstrate safety and effectiveness in a larger group of dogs.
How Gallant’s Research Works
Gallant’s investigational stem cell products are manufactured under Current Good Manufacturing Practices (cGMP) and evaluated according to FDA-regulated protocols. Each batch of cells undergoes rigorous quality and potency testing before being used in clinical trials.
These studies are designed to explore whether stem cell therapy can be a practical, science-based option that veterinarians may one day integrate into everyday care for conditions like atopic dermatitis.

Looking Ahead
Chronic skin disease can be frustrating—for pets, for veterinarians, and for the families who love them. Regenerative medicine represents a new area of scientific exploration that focuses on restoring balance rather than masking symptoms.
While stem cell therapy for atopic dermatitis remains investigational, Gallant’s work is helping to advance understanding of how these cells may support healthy immune function in the future.
Learn More
Gallant is leading the next generation of veterinary regenerative medicine, developing ready-to-use stem cell therapies designed to help pets live longer, healthier, and more comfortable lives.
Visit our Clinical Trials page
Learn more about participation and find a clinic near you.
All participation is voluntary and conducted under veterinary supervision in accordance with FDA regulations.
Disclaimer
Gallant’s investigational stem cell therapies are not commercially available. These veterinary products may be available through participation in a study at a qualified clinic under FDA-authorized protocols. This blog is intended for educational purposes only and does not constitute medical advice.


