Stem Cell Therapy for Feline Chronic Gingivostomatitis (FCGS): A New Path to Healing
What pet parents should know about this therapy for Feline Chronic Gingivostomatitis
Feline Chronic Gingivostomatitis (FCGS) is a painful and frustrating condition—both for the cats who live with it and the humans who love them. If your cat has been diagnosed, you’ve probably tried it all: tooth extractions, antibiotics, steroids. And yet, the flare-ups persist. The discomfort continues.
At Gallant, we’re working to change that.
We’re studying a new class of care—stem cell therapy—with the goal of helping veterinarians and pet parents explore new ways to support cats living with FCGS. This blog explores what we’re learning and why there’s reason to be hopeful.
Note: Gallant’s investigational therapies are not approved for commercial use. All studies are conducted under FDA oversight.
What Is FCGS?
Feline Chronic Gingivostomatitis (FCGS) is a severe oral inflammatory condition that causes significant pain and inflammation in a cat’s mouth. It often leads to difficulty eating, grooming, and engaging in daily activities.
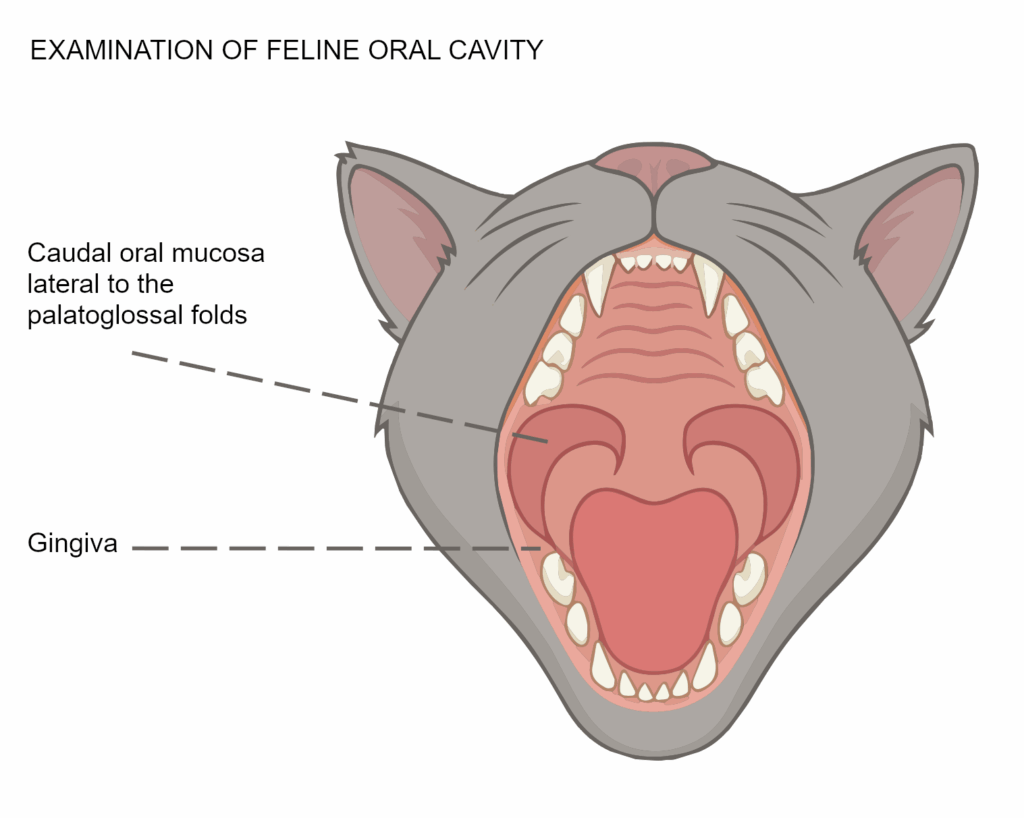
Common symptoms include:
- Red, inflamed, or ulcerated gums
- Difficulty eating or chewing
- Drooling or bad breath
- Weight loss and lethargy
- Vocalization due to oral pain
For many pet parents, treatment means a long journey through dental extractions, medications, and repeated flare-ups—with no clear path to resolution.
A New Approach: Investigating Stem Cell Therapy
Gallant is developing an off-the-shelf stem cell therapy for cats with FCGS. This therapy is currently being evaluated under an FDA-authorized clinical trial. It’s part of a new class of care called regenerative medicine—designed to help restore function and support the body’s natural repair mechanisms.
Here’s what makes our investigational therapy different:
- High stem cell quality: Stem cells are sourced from the uterine tissue of healthy FDA-qualified donors during routine spay procedures, meaning your pet doesn’t undergo any unnecessary anesthesia or surgery to source the stem cells
- Ready to use: Our stem cell products are shipped on-demand, limiting long wait times
- Regulated and standardized: Developed with quality controls in a Good Manufacturing Practices-compliant facility, in alignment with the FDA’s structured review and approval process.
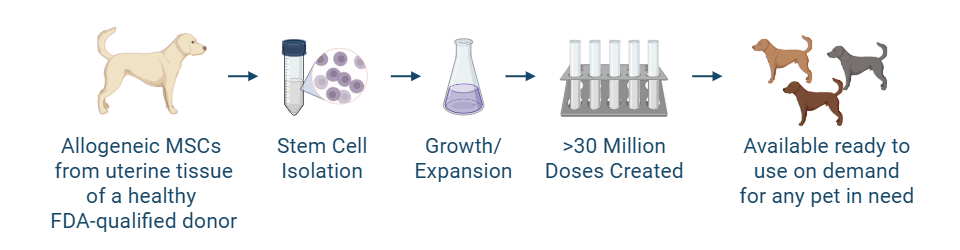
While still under investigation, this therapy is being explored as a potential option for cases that don’t respond to traditional care.
How to Get Involved
Gallant is currently enrolling cats into clinical trials at veterinary practices across the country. If your cat has been diagnosed with FCGS and you’re seeking new options, talk to your veterinarian about whether a clinical study may be appropriate.
Visit our Clinical Trials page
Learn more about participation and find a clinic near you.
All participation is voluntary and conducted under veterinary supervision in accordance with FDA regulations.
Why Gallant?
Gallant is leading the development of regulated stem cell therapies for pets. Founded by experts in veterinary regenerative medicine, we are committed to developing ethical, science-backed solutions that go beyond managing symptoms—and aim to restore health at the source.
Our focus is clear: help pets live better, more vibrant lives. Because every extra year should be a good one.
Disclaimer
Gallant’s investigational stem cell therapies are not commercially available. These veterinary products may be available through participation in a study at a qualified clinic under FDA-authorized protocols. This blog is intended for educational purposes only and does not constitute medical advice.


