Disease State
Clinical definition
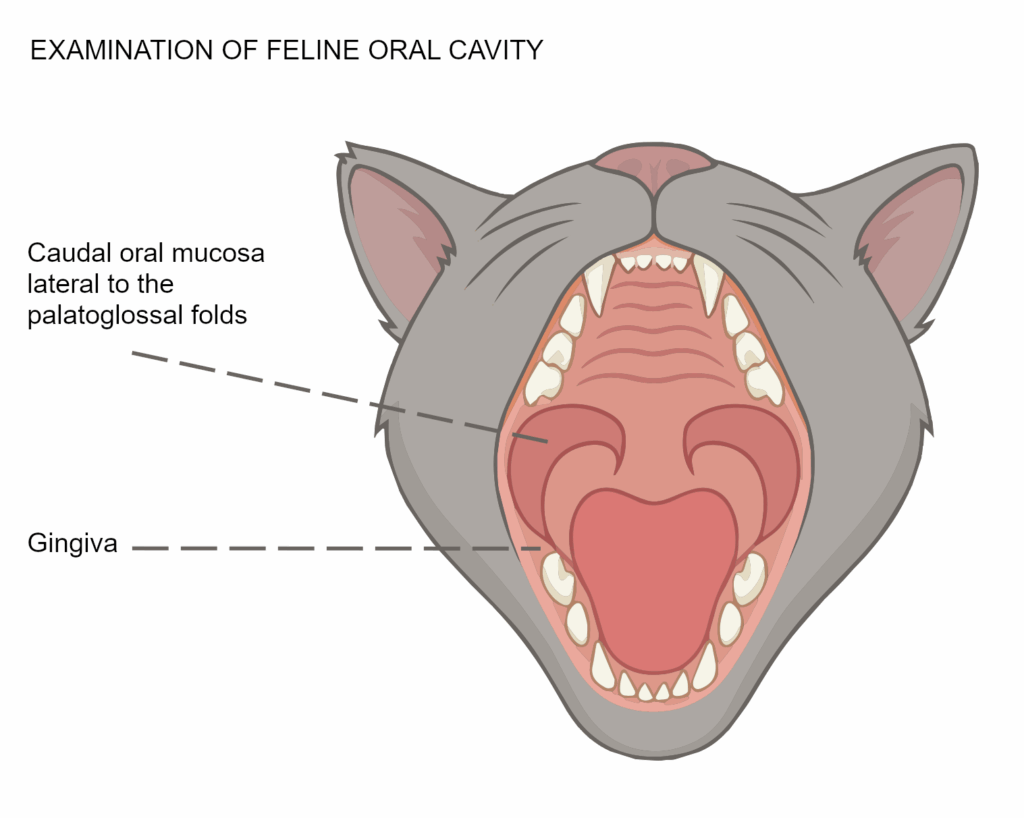
Feline Chronic Gingivostomatitis (FCGS), also known as chronic stomatitis, is a complex disease characterized by oral inflammation with an immune-mediated component and elusive etiology. Oral inflammation typically involves areas of the gingiva, alveolar and buccal mucosa, as well as the caudal oral mucosa, lateral to the palatoglossal folds. It is a painful, chronic, and debilitating inflammatory disease.
Etiology/cause
It is generally recognized that FCGS is the result of overactive and sustained T-cell expansion and T-cell exhaustion, both systemically and within the oral cavity. This was initially thought to be due to an abnormal response to plaque; however, it is now clear that the true cause is not completely elucidated, as the pathogenesis and progression differ from case to case.

Factors

Disease progression is multifactorial and often involves co-morbidity with infectious agents such as feline calicivirus, feline herpesvirus, feline leukemia virus, feline immunodeficiency virus, and Bartonella henselae, with feline calicivirus thought to be the primary infectious disease agent associated with FCGS. Additional co-morbidities include tooth resorption (feline odontoclastic resorption lesions/FORL), periodontal disease, food allergies, and hypersensitivity to plaque-forming bacteria.
Immune dysregulation
Immune dysregulation in FCGS occurs both locally and systemically. Locally, there is increased expression of mRNA for specific inflammatory mediators (IL-2, IL-4, IL-6, IL-10, IL-12, and IFN-γ). The predominant cells infiltrating the caudal oral mucosa in affected cats are primarily CD79a+ IgG isotype plasma cells, neutrophils (L1+ cells), and CD8+ (cytotoxic) T cells. Systemically, the cytokine expression profile mimics what is observed locally and is characterized by elevated circulating levels of IFN-γ, TNF-α, and IL-lβ, neutrophilia in 30-40% of patients, as well as CD8+ effector memory cells.

Medical management
Stimulation of the immune system by plaque bacteria appears to contribute to ongoing inflammation, and successful treatment of chronic stomatitis requires minimizing oral bacteria. Daily plaque removal by mechanical means (e.g., toothbrushing) is difficult in these painful cats, and reduction of plaque-retentive surfaces by extracting teeth has proven to be most effective in eliminating plaque and reducing oral inflammation. Antibiotics may be used as medically indicated.
Analgesics (opioids such as buprenorphine), gabapentin, non-steroidal anti-inflammatories, and immunosuppressive medications, including cyclosporine and corticosteroids (prednisone or prednisolone), are often used alone or in combination to manage pain and inflammation. Medical management without tooth extraction is likely to only temporarily alleviate clinical signs. Response to medical therapy alone is only observed in 23-45% of cases and is mostly transient.
Surgical management
The surgical approach to FCGS includes the removal of all teeth that have abnormalities on oral examination and imaging. Complete removal, including the roots, is important and should be confirmed with post-operative imaging.
Refractory FCGS
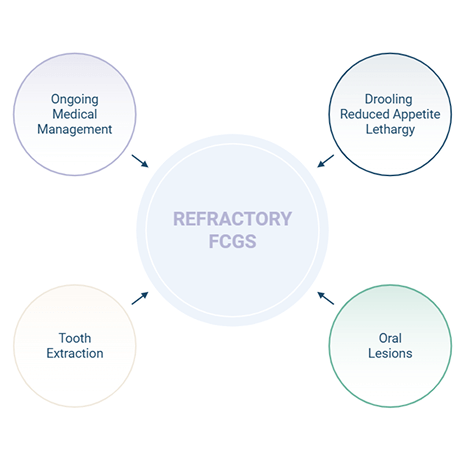
Of the cats with FCGS that have full-mouth extractions, 50-80% of cats will significantly improve. However, for the remaining 20-50%, abnormal clinical signs, loss of quality of life, and oral inflammation persist, requiring lifelong management. Continued clinical signs and oral inflammation despite the extraction of affected teeth (at least 2 months prior) and medical management are considered refractory (nonresponsive).
Unmet medical need
Cats suffering from refractory FCGS are often faced with lifelong anti-inflammatory and immunosuppressive drug therapy. There are no FDA-approved medications, and the medications that are currently used have no proven safety or effectiveness in the long-term management of FCGS and have highly variable clinical response rates.
Especially in cats, the available medications are also associated with potentially significant peripheral organ damage and toxicity, resulting in potential side effects that may not be well tolerated by the patient (examples include vomiting, bloody diarrhea, anorexia, frequent urination, vocalization, etc.). If the cats do not have some clinical control of inflammation and pain with these drugs, they may also experience reduced food intake, weight loss, lethargy, and overall ill thrift. The outcome for the unresponsive cat is a decreased quality of life and decreased life span due to death or euthanasia.
Many of these signs may make it difficult for the owner to be compliant with giving medications, which may require daily administration of oral medication, creating unwanted social behaviors and challenging the human-animal bond.
Why stem cells for the treatment of rFCGS
Mesenchymal stem cells, referred to as MSCs, are the body’s adult repair cells, allowing healing and restoration of damaged tissues naturally. MSCs are multipotent stem cells with differentiation limited to certain cell lineages and are capable of interacting with and modulating various immune cell subsets, such as T cells. Stem cells offer a therapeutic approach that targets the body’s natural healing mechanisms to heal diseased tissues rather than just control clinical signs. Stem cells work through mechanisms that spare organ damage in non-targeted tissues.

Diagnosis
Clinical signs/chief complaint

Cats present with clinical signs characteristic of oral disease, including bad breath (halitosis), poor or reduced grooming activities, difficulty eating, reduced appetite or inappetence, pain when eating or yawning, weight loss, and drooling. Additional clinical signs signaling reduced quality of life and general ill health include: irritability, hiding, vocalizing when eating, and reduced socialization with other pets and family members.
Differential diagnosis
There are a number of oral diseases that may mimic or have similar clinical signs to FCGS.
- Periodontal disease: FCGS may be differentiated from periodontal disease by the presence of inflammatory lesions affecting tissues beyond the gingiva, including the caudal oral mucosa.
- Eosinophilic granuloma complex: FCGS may be differentiated from eosinophilic granuloma complex histologically.

- Neoplasia: FCGS may be differentiated from squamous cell carcinoma (SCC) and other oral neoplasia histologically. It is possible for FCGS to present as proliferative and can transform into SCC, therefore, accurate diagnosis in these cases with a fine needle aspirate or biopsy is important for prognosis and treatment.
- Gingivitis: Similar to periodontal disease, FCGS may be differentiated from gingivitis by the presence of inflammatory lesions affecting tissues beyond the gingiva, including the caudal oral mucosa. Other tissues in the oral cavity are affected in FCGS.
- Other stomatitis (i.e., autoimmune causes, paraneoplastic, electrical/thermal/ chemical burn)
These diagnoses may require additional diagnostics to confirm, and a good clinical history may rule out additional causes.
Diagnostics
Oral exam:
- An oral examination should identify inflammation that crosses the mucogingival junction, involving the gingiva and extending to the buccal and caudal oral mucosa, lateral to the palatoglossal folds.
- Lesion scores.
Two observed phenotypes:
- ulcerative/erosive, and/or
- proliferative
A combination of both is possible.

CLINICAL PATHOLOGY
Complete blood count, serum biochemistry panel, and urinalysis should be performed to assess the patient’s overall health status and as a pre-operative health assessment if surgical care is recommended.

INFECTIOUS DISEASE TESTING
Viral disease testing to assess FeLV, FIV, and FCV status should be performed due to the potential prognostic significance and to document potential causative factors.

HISTOPATHOLOGY
Histological characteristics of FCGS include Infiltrates of lymphocytes and a cells, with varying numbers of neutrophils.
Lesion Scoring Chart
Score the inflammation of oral cavity sites:
0 = none; normal pink, healthy tissue
1 = mild; mild erythema, likely no ulceration or proliferation
2 = moderate; moderate erythema, superficial erosion or ulceration, moderate proliferation, no spontaneous hemorrhage
3 = severe; extreme erythema with spontaneous hemorrhage, deep ulceration and/or significant proliferation of tissues

TOTAL SCORE (MAXIMUM = 24):
Mechanism of Action
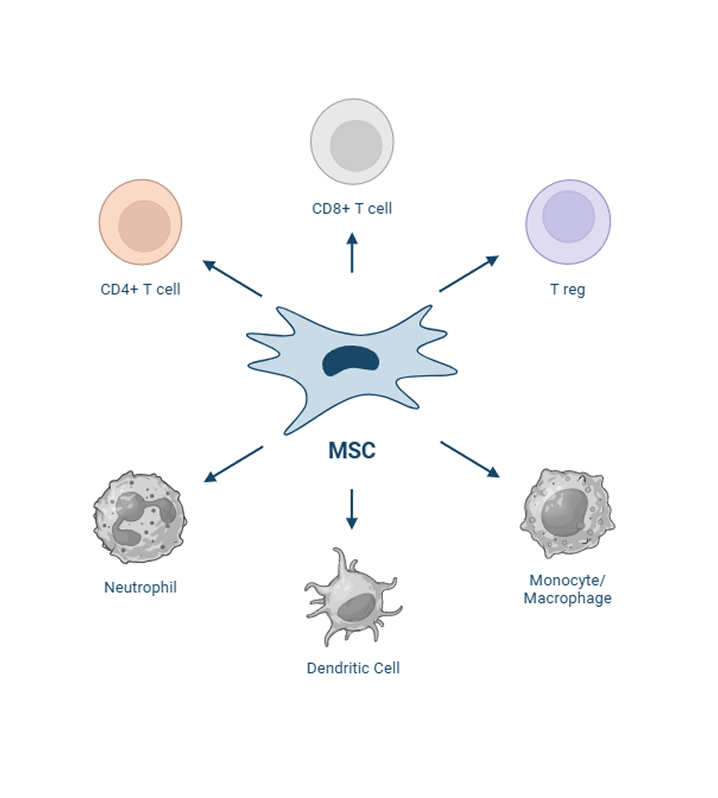
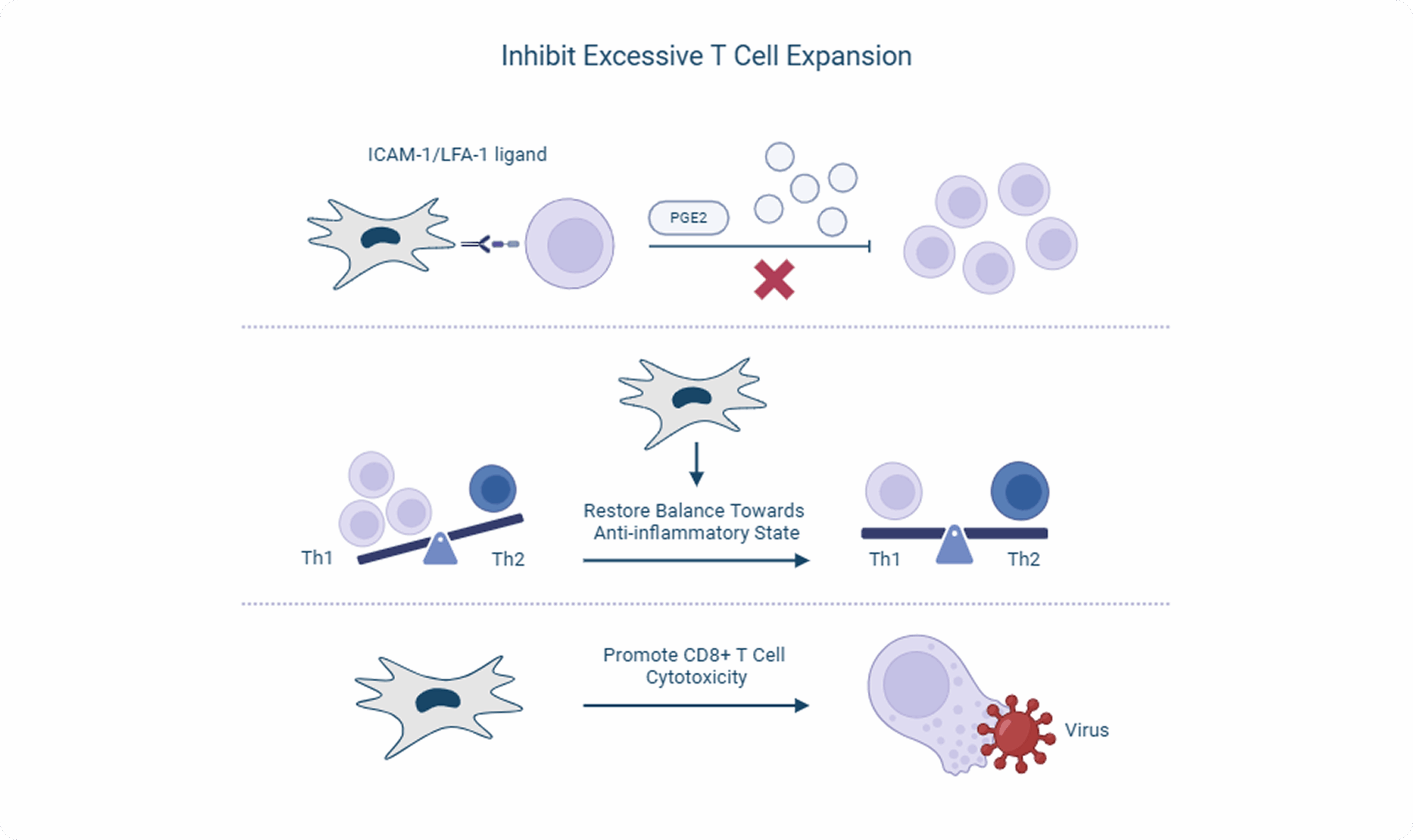
Stem cells are administered intravenously, where they are introduced into the bloodstream and can recognize and react to the microenvironment, particularly inflammation, both directly, through cellular interactions with immune cells (T-cells, macrophages, B cells), and indirectly through paracrine signaling (interleukins, TGF, etc). The investigational uterine-derived MSCs do not elicit an immune response in the recipient animal because they do not express MHC II on their cell surface.
Once administered, the MSCs are capable of homing to sites of inflammation as a response to chemotactic cytokines secreted by inflammatory cells such as TNF-alpha and interferon-gamma. In response to their environment, the cells produce several anti-inflammatory paracrine factors to mitigate excessive inflammation, mainly prostaglandin-E2 and indoleamine 2,3 dioxygenase, but also hepatocyte growth factor, tumor necrosis factor-stimulated gene 6, interleukin-6, and tumor growth factor-β. These secreted factors limit immune cell expansion. The cells also act to restore the balance of dysregulated T-helper cell subtypes so that the Th-2 cells are no longer dominant.
In addition, a few pathogens have been shown to be concomitant with FCGS, particularly feline calicivirus or FCV but also feline leukemia virus and feline foamy virus. The investigational uterine-derived MSCs appear to aid in viral clearance of FCV-positive cats, likely due to enhancement of the cytotoxicity capability of CD8+ T cells.
Cellular Interactions Specific To FCGS
Uterine derived MSCs
The endometrium is the lining of the uterus, and it undergoes cyclic regeneration during each estrous cycle. As a result, it is a readily accessible and renewable source of stem cells. Obtaining allogeneic uterine-derived MSCs is less invasive and less complicated than other sources, making this cell source more feasible for therapeutic applications. Uterine-derived MSCs have also been found to possess potent immunomodulatory properties. They can regulate the immune response and reduce inflammation, crucial for treating chronic oral inflammatory diseases.
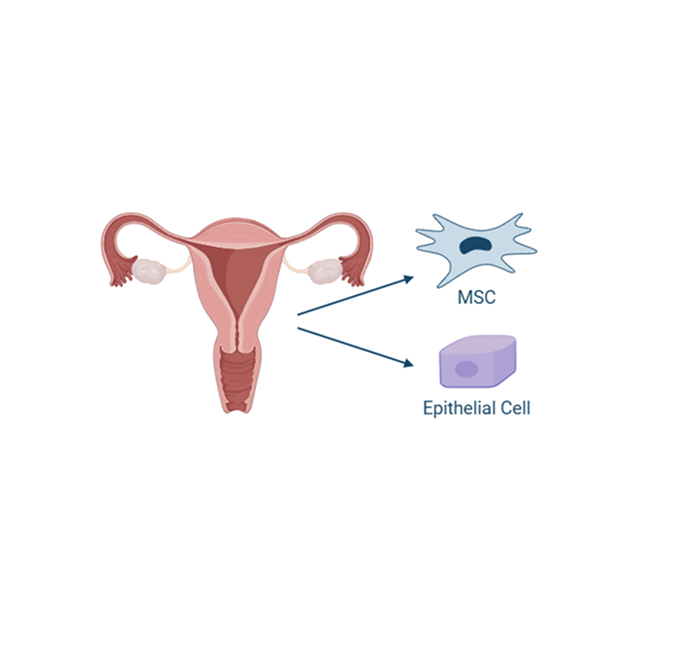
Additionally, MSCs derived from the uterustend to have low expression of major histocompatibility complex (MHC) antigens, which makes them less likely to be recognized and attacked by the recipient’s immune system.
Consequently, this treatment may improve the success rate of extractions and become a more readily available source for these cells. This approach will revolutionize the treatment options for cats with feline chronic gingivostomatitis (FCGS), enhance their quality of life, and expand cell therapy to a broader range of diseased cats. The stem cells under investigational use for refractory FCGS are feline allogeneic uterine-derived from the perivascular area and the endometrial layer of the uterus from a healthy FDA-qualified donor.
A deeper look into the life organ
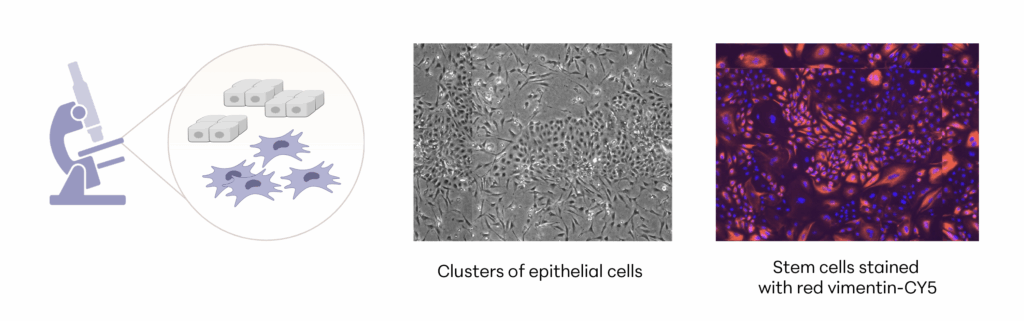
Manufacturing and Potency
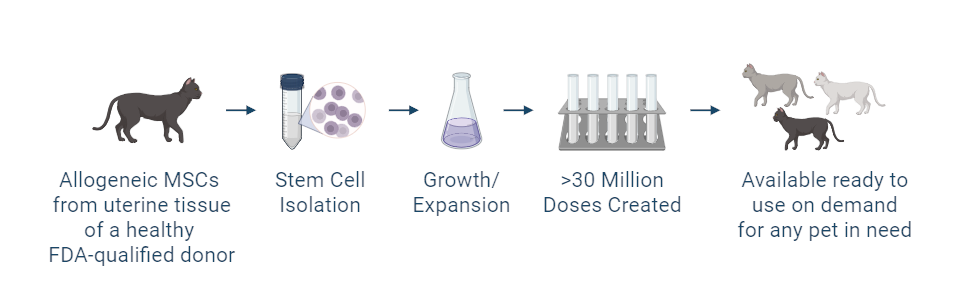
The investigational stem cells being studied are collected from a FDA-qualified feline (cat) donor during a routine spay procedure. The cells are intended to be used in other cats (same species) as an allogeneic ready-to-use product. The uterine-derived MSCs have been demonstrated in a matrix of assays to be potent (functional) and directed at the relevant clinical factors in FCGS. Each batch of stem cells is manufactured according to Current Good Manufacturing Practices (CGMP) and is released with established specifications demonstrating key quality attributes for identity, purity, safety and potency of the drug product.
Allogeneic cells from a FDA qualified donor
References
- Soltero-Rivera M, Goldschmidt S, Arzi B. Feline chronic gingivostomatitis current concepts in clinical management. Journal of Feline Medicine and Surgery. 2023;25(8). doi:10.1177/1098612X231186834
- Verhaert L, Van Wetter C. Survey of oral diseases in cats in Flanders. Vlaams Diergeneeskundig Tijdschrift 2004; 73: 331-341. ISI.
- Healey KAE, Dawson S, Burrow R, et al. Prevalence of feline chronic gingivo-stomatitis in first opinion veterinary practice. J Feline Med Surg 2007; 9: 373-381. Crossref. PubMed. ISI.
- Girard N, Servet E, Biourge V, et al. Periodontal health status in a colony of 109 cats. J Vet Dent 2009; 26: 147-155. Crossref. PubMed. ISI.
- Kim D-H, Kwak H-H, Woo H-M. Prevalence of feline chronic gingivostomatitis in feral cats and its risk factors. J Feline Med Surg 2023; 25. Crossref.
- Jennings MW, Lewis JR, Soltero-Rivera MM, et al. Effect of tooth extraction on stomatitis in cats: 95 cases (2000-2013). J Am Vet Med Assoc 2015; 246; 654-660. Crossref. PubMed.
- Bellei E, Dalla F, Masetti L, et al. Surgical therapy in chronic feline gingivostomatitis (FCGS). Vet Res Commun 2008; 32: 231-234. Crossref. PubMed. ISI.
- Druet I, Hennet P. Relationship between feline cali-civirus load, oral lesions, and outcome in feline chronic gingivostomatitis (caudal stomatitis): retrospective study in 104 cats. Front Vet Sci 2017; 4: 209. Crossref. PubMed.
- Vapniarsky N, Simpson DL, Arzi B, et al. Histological, immunological, and genetic analysis of feline chronic gingivostomatitis. Front Vet Sci 2020; 7: 310. Crossref. PubMed.
- Rodrigues MX, Bicalho RC, Fiani N, et al. The subgingival microbial community of feline periodontitis and gingivo-stomatitis: characterization and comparison between diseased and healthy cats. Sci Rep 2019; 9: 12340. Crossref. PubMed.
- Fried WA, Soltero-Rivera M, Ramesh A, et al. Use of unbiased metagenomic and transcriptomic analyses to investigate the association between feline calicivirus and feline chronic gingivostomatitis in domestic cats. Am J Vet Res 2021; 82:381-394. Crossref. PubMed.
- Peralta S, Grenier JK, Webb SM, et al. Transcriptomic signatures of feline chronic gingivostomatitis are influenced by upregulated IL6 [preprint]. Res Sq 2023. Crossref. PubMed.
- Rodrigues MX, Fiani N, Bicalho RC, et al. Preliminary functional analysis of the subgingival microbiota of cats with periodontitis and feline chronic gingivostomatitis. Sci Rep 2021; 11: 6896. Crossref. PubMed.
- Ruparell A, Inui T, Staunton R, et al. The canine oral micro-biome: variation in bacterial populations across different niches. BMC Microbiol 2020; 20: 42. Crossref. PubMed.
- Nanci A. Oral mucosa. In: Ten Cate’s oral histology. 9th ed. St Louis, MO: Elsevier, 2018, pp 260-288.
- Murphy BG, Bell CM, Soukup JW (eds). Histologic features of normal oral tissues. In: Veterinary oral and maxillofacial pathology. Hoboken, NJ: Wiley, 2020; pp 3-10.
- Wu R-Q, Zhang D-F, Tu E, et al. The mucosal immune system in the oral cavity – an orchestra of T cell diversity. Int J Oral Sci 2014; 6: 125-132. Crossref. PubMed. ISI.
- Moutsopoulos NM, Konkel JE. Tissue-specific immunity at the oral mucosal barrier. Trends Immunol 2018; 39: 276-287. Crossref. PubMed.
- §enel S. An overview of physical, microbiological and immune barriers of oral mucosa. Int J Mol Sci 2021; 22: 7821. Crossref. PubMed.
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Ann Rev Immunol 2004; 22: 745-763. Crossref. PubMed. ISI.
- Arzi B, Murphy B, Baumgarth N, et al. Analysis of immune cells within the healthy oral mucosa of specific pathogen-free cats. Anat Histol Embryol 2011; 40: 1-10. Crossref. PubMed.
- Harley R, Gruffydd-Jones TJ, Day MJ. Immuno -histochemical characterization of oral mucosal lesions in cats with chronic gingivostomatitis. J Comp Pathol 2011; 144: 239-250. Crossref. PubMed. ISI.
- Arzi B, Murphy B, Cox DP, et al. Presence and quantification of mast cells in the gingiva of cats with tooth resorption, periodontitis and chronic stomatitis. Arch Oral Biol 2010; 55: 148-154. Crossref. PubMed. ISI.
- Harley R, Helps CR, Harbour DA, et al. Cytokine mRNA expression in lesions in cats with chronic gingivostomatitis. Clin Diagn Lab Immunol 1999; 6: 471-478. Crossref. PubMed.
- Farcas N, Lommer MJ, Kass PH, et al. Dental radiographic findings in cats with chronic gingivostomatitis (2002-2012). J Am Vet Med Assoc 2014; 244: 339-345. Crossref. PubMed.
- Lommer MJ. Oral inflammation in small animals. Vet Clin North Am Small Anim Pract 2013; 43: 555-571. Crossref. PubMed. ISI.
- Mestrinho LA, Rosa R, Ramalho P, et al. A pilot study to evaluate the serum alpha-1 acid glycoprotein response in cats suffering from feline chronic gingivostomatitis. BMC Vet Res 2020; 16: 390. Crossref. PubMed.
- White SD, Rosychuk RA, Janik TA, et al. Plasma cell stomatitis-pharyngitis in cats: 40 cases (1973-1991). J Am Vet Med Assoc 1992; 200: 1377-1380. Crossref. PubMed. ISI.
- Harley R, Gruffydd-Jones TJ, Day MJ. Determination of salivary and serum immunoglobulin concentrations in the cat. Vet Immunol Immunopathol 1998; 65: 99-112. Crossref. PubMed.
- Arzi B, Mills-Ko E, Verstraete FJ, et al. Therapeutic efficacy of fresh, autologous mesenchymal stem cells for severe refractory gingivostomatitis in cats. Stem Cells Transl Med 2016; 5: 75-86. Crossref. PubMed. ISI.
- Kouki MI, Papadimitriou SA, Psalla D, et al. Chronic gin-givostomatitis with esophagitis in cats. J Vet Intern Med 2017; 31: 1673-1679. Crossref. PubMed.
- Lommer MJ, Verstraete FJM. Concurrent oral shedding of feline calicivirus and feline herpesvirus 1 in cats with chronic gingivostomatitis. Oral Microbiol Immunol 2003; 18: 131-134. Crossref. PubMed.
- Dolieslager SMJ, Bennett D, Johnston N, et al. Novel bacterial phylotypes associated with the healthy feline oral cavity and feline chronic gingivostomatitis. Res Vet Sci 2013; 94: 428-432.
- Quimby JM, Elston T, Hawley J, et al. Evaluation of the association of Bartonella species, feline herpesvirus 1, feline calicivirus, feline leukemia virus and feline immunodeficiency virus with chronic feline gingivostomatitis. J Feline Med Surg 2008; 10: 66-72. Crossref. PubMed. ISI.
- Dolieslager SM, Lappin DF, Bennett D, et al. The influence of oral bacteria on tissue levels of Toll-like receptor and cytokine mRNAs in feline chronic gingivostomatitis and oral health. Vet Immunol Immunopathol 2013; 151: 263-274. Crossref. PubMed.
- Knowles JO, McArdle F, Dawson S, et al. Studies on the role of feline calicivirus in chronic stomatitis in cats. Vet Microbiol 1991; 27: 205-219. Crossref. PubMed. ISI.
- Reubel GH, Hoffmann DE, Pedersen NC. Acute and chronic faucitis of domestic cats: a feline calicivirus-induced disease. Vet Clin North Am Small Anim Pract 1992; 22: 1347-1360. Crossref. PubMed. ISI.
- Addie DD, Radford A, Yam PS, et al. Cessation of feline calicivirus shedding coincident with resolution of chronic gingivostomatitis in a cat. J Small Anim Pract 2003; 44: 172-176. Crossref. PubMed. ISI.
- Matsumoto H, Teshima T, Iizuka Y, et al. Evaluation of the efficacy of the subcutaneous low recombinant feline interferon-omega administration protocol for feline chronic gingivitis-stomatitis in feline calicivirus-positive cats. Res Vet Sci 2018; 121: 53-58. Crossref. PubMed.
- Tenorio AP, Franti CE, Madewell BR, et al. Chronic oral infections of cats and their relationship to persistent oral carriage of feline calici-, immunodeficiency, or leukemia viruses. Vet Immunol Immunopathol 1991; 29: 1-14. Crossref. PubMed. ISI.
- Silva M, Fernandes M, Fialho M, et al. A case series analysis of dental extractions’ outcome in cats with chronic gingivo-stomatitis carrying retroviral disease. Animals (Basel) 2021; 11: 3306. Crossref. PubMed.
- Peralta S, Carney PC. Feline chronic gingivostomatitis is more prevalent in shared households and its risk correlates with the number of cohabiting cats. J Feline Med Surg 2019; 21: 1165-1171. Crossref. PubMed. ISI.
- Little S, Levy J, Hartmann K, et al. 2020 AAFP feline retro-virus testing and management guidelines. J Feline Med Surg 2020; 22: 5-30. Crossref. PubMed. ISI.
- Hofmann-Lehmann R, Hosie MJ, Hartmann K, et al. Calicivirus infection in cats. Viruses 2022; 14: 937. Crossref. PubMed.
- Lommer MJ. Efficacy of cyclosporine for chronic, refractory stomatitis in cats: a randomized, placebo-controlled, double-blinded clinical study. J Vet Dent 2013; 30: 8-17. Crossref. PubMed. ISI.
- Arzi B, Peralta S, Fiani N, et al. A multicenter experience using adipose-derived mesenchymal stem cell therapy for cats with chronic, non-responsive gingivostomatitis. Stem Cell Res Ther 2020; 11: 115. Crossref. PubMed.
- Arzi B, Clark KC, Sundaram A, et al. Therapeutic efficacy of fresh, allogeneic mesenchymal stem cells for severe refractory feline chronic gingivostomatitis. Stem Cells Transl Med 2017; 6: 1710-1722. Crossref. PubMed. ISI.
- Hennet PR, Camy GAL, McGahie DM, et al. Comparative efficacy of a recombinant feline interferon omega in refractory cases of calicivirus-positive cats with caudal stomatitis: a randomised, multi-centre, controlled, double-blind study in 39 cats. J Feline Med Surg 2011; 13: 577-587. Crossref. PubMed. ISI.
- McTavish S. Eosinophilic gastroenteritis in a dog. Can Vet J 2002; 43: 463-465. PubMed.
- Konstantinidis AO, Mylonakis ME, Psalla D, et al. Pyloric obstruction due to massive eosinophilic infiltration in a young adult dog. Can Vet J 2017; 58: 1164-1166. PubMed.
- Rousseau M. Severe lymphocytic-plasmacytic and atrophic gastritis, as well as, predominantly eosinophilic, severe enteritis, in a 19-month-old Labrador Retriever. Can Vet J 2005; 46: 264-267. PubMed.
- Linton M, Nimmo JS, Norris JM, et al. Feline gastrointestinal eosinophilic sclerosing fibroplasia: 13 cases and review of an emerging clinical entity. J Feline Med Surg 2015; 17: 392-404. Crossref. PubMed. ISI.
- Hendrick M. A spectrum of hypereosinophilic syndromes exemplified by six cats with eosinophilic enteritis. Vet Pathol 1981; 18: 188-200. Crossref. PubMed. ISI.
- Hennet P. Chronic gingivo-stomatitis in cats: long-term follow-up of 30 cases treated by dental extractions. J Vet Dent 1997; 14: 15-21. Crossref.
- Reiter AM, Soltero-Rivera MM. Applied feline oral anatomy and tooth extraction techniques: an illustrated guide. J Feline Med Surg 2014; 16: 900-913. Crossref. PubMed. ISI.
- Stathopoulou T-R, Kouki M, Pypendop BH, et al. Evaluation of analgesic effect and absorption of buprenorphine after buccal administration in cats with oral disease. J Feline Med Surg 2018; 20: 704-710. Crossref. PubMed. ISI.
- Shipley H, Flynn K, Tucker L, et al. Owner evaluation of quality of life and mobility in osteoarthritic cats treated with amantadine or placebo. J Feline Med Surg 2021; 23: 568-574. Crossref. PubMed. ISI.
- Siao KT, Pypendop BH, Stanley SD, et al. Pharmacokinetics of amantadine in cats. J Vet Pharmacol Ther 2011; 34: 599-604. Crossref. PubMed.
- Cheng J-K, Chiou L-C. Mechanisms of the antinociceptive action of gabapentin. J Pharmacol Sci 2006; 100: 471-486. Crossref. PubMed. ISI.
- Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA 1998; 280: 1831-1836. Crossref. PubMed. ISI.
- Adrian DE, Rishniw M, Scherk M, et al. Prescribing practices of veterinarians in the treatment of chronic musculoskeletal pain in cats. J Feline Med Surg 2019; 21: 495-506. Crossref. PubMed. ISI.
- Pypendop BH, Siao KT, Ilkiw JE. Thermal antinociceptive effect of orally administered gabapentin in healthy cats. Am J Vet Res 2010; 71: 1027-1032. Crossref. PubMed. ISI.
- Aghighi SA, Tipold A, Piechotta M, et al. Assessment of the effects of adjunctive gabapentin on postoperative pain after intervertebral disc surgery in dogs. Vet Anaesth Analg 2012; 39: 636-646. Crossref. PubMed.
- Steagall PV, Benito J, Monteiro BP, et al. Analgesic effects of gabapentin and buprenorphine in cats undergoing ovario-hysterectomy using two pain-scoring systems: a randomized clinical trial. J Feline Med Surg 2018; 20: 741-748. Crossref. PubMed. ISI.
- Siao KT, Pypendop BH, Ilkiw JE. Pharmacokinetics of gabapentin in cats. Am J Vet Res 2010; 71: 817-821. Crossref. PubMed. ISI.
- Mensah-Nyagan AG, Meyer L, Schaeffer V, et al. Evidence for a key role of steroids in the modulation of pain. Psychoneuroendocrinol 2009; 34 Suppl 1: S169-S177. Crossref. PubMed.
- Blank CU, Haining WN, Held W, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol 2019; 19: 665-674. Crossref. PubMed.
- King JN, King S, Budsberg SC, et al. Clinical safety of robenacoxib in feline osteoarthritis: results of a randomized, blinded, placebo-controlled clinical trial. J Feline Med Surg 2016; 18: 632-642. Crossref. PubMed. ISI.
- Gowan RA, Lingard AE, Johnston L, et al. Retrospective case-control study of the effects of long-term dosing with meloxi-cam on renal function in aged cats with degenerative joint disease. J Feline Med Surg 2011; 13: 752-761. Crossref. PubMed. ISI.
- Gowan RA, Baral RM, Lingard AE, et al. A retrospective analysis of the effects of meloxicam on the longevity of aged cats with and without overt chronic kidney disease. J Feline Med Surg 2012; 14: 876-881. Crossref. PubMed. ISI.
- Hung Y-P, Yang YP, Wang HC, et al. Bovine lactoferrin and piroxicam as an adjunct treatment for lymphocytic-plasma-cytic gingivitis stomatitis in cats. Vet J 2014; 202: 76-82. Crossref. PubMed.
- Sato R, Inanami O, Tanaka Y, et al. Oral administration of bovine lactoferrin for treatment of intractable stomatitis in feline immunodeficiency virus (FlV)-positive and FIV-negative cats. Am J Vet Res 1996; 57: 1443-1446. PubMed. ISI.
- Harvey CE, Thornsberry C, Miller BR, et al. Antimicrobial susceptibility of subgingival bacterial flora in cats with gingivitis. J Vet Dent 1995; 12: 157-160. Crossref. PubMed.
- Lodi G, Azzi L, Varoni EM, et al. Antibiotics to prevent complications following tooth extractions. Cochrane Database Syst Rev 2021; CD003811. Crossref. PubMed.
- Hilchey SP, Palshikar MG, Emo JA, et al. Cyclosporine a directly affects human and mouse b cell migration in vitro by disrupting a hIF-1a dependent, o2 sensing, molecular switch. BMC Immunol 2020; 21: 13. Crossref. PubMed.
- Vercelli A, Raviri G, Cornegliani L. The use of oral cyclosporin to treat feline dermatoses: a retrospective analysis of 23 cases. Vet Dermatol 2006; 17: 201-206. Crossref. PubMed. ISI.
- de Mari K, Maynard L, Sanquer A, et al. Therapeutic effects of recombinant feline interferon-omega on feline leukemia virus (FeLV)-infected and FeLV/feline immunodeficiency virus (FIV)-coinfected symptomatic cats. J Vet Intern Med 2004; 18: 477-482. Crossref. PubMed. ISI.
- Gil S, Leal RO, McGahie D, et al. Oral recombinant feline interferon-omega as an alternative immune modulation therapy in FIV positive cats: clinical and laboratory evaluation. Res Vet Sci 2014; 96: 79-85. Crossref. PubMed. ISI.
- Gil S, Leal RO, Duarte A, et al. Relevance of feline interferon omega for clinical improvement and reduction of concurrent viral excretion in retrovirus infected cats from a rescue shelter. Res Vet Sci 2013; 94: 753-763. Crossref. PubMed. ISI.
- Rivas IL, Soltero-Rivera M, Vapniarsky N, et al. Stromal cell therapy in cats with feline chronic gingivostomatitis: current perspectives and future direction. J Feline Med Surg 2023; 25. Crossref. PubMed.
- Arzi B, Taechangam N, Lommer MJ, et al. Stem cell therapy prior to full-mouth tooth extraction lacks substantial clinical efficacy in cats affected by chronic gingivostomatitis. J Feline Med Surg 2021; 23: 604-608. Crossref. PubMed. ISI.
- Franco F, Jaccard A, Romero P, et al. Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab 2020; 2: 1001-1012. Crossref. PubMed.
- Weber MG, Walters-Laird CJ, Kol A, et al. Gut germinal center regeneration and enhanced antiviral immunity by mesenchymal stem/stromal cells in SIV infection. JCI Insight 2021; 6. Crossref.
- Taechangam N, Kol A, Arzi B, et al. Multipotent stromal cells and viral interaction: current implications for therapy. Stem Cell Rev Rep 2022; 18: 214-227. Crossref. PubMed.
- Castelli V, Lombardi A, Palomba E, et al. Immune checkpoint inhibitors in people living with HIV/AIDS: facts and controversies. Cells 2021; 10: 2227. Crossref. PubMed.
- Hoogeveen RC, Boonstra A. Checkpoint inhibitors and therapeutic vaccines for the treatment of chronic HBV infection. Front Immunol 2020; 11. Crossref. PubMed.
- Leñero C, Kaplan LD, Best TM, Kouroupis D. CD146+ Endometrial-Derived Mesenchymal Stem/Stromal Cell Subpopulation Possesses Exosomal Secretomes with Strong Immunomodulatory miRNA Attributes. Cells. 2022 Dec 10;11(24):4002. doi: 10.3390/cells11244002. PMID: 36552765; PMCID: PMC9777070.
- Cheng Y, Li L, Wang D, Guo Q, He Y, Liang T, Sun L, Wang X, Cheng Y, Zhang G. Characteristics of Human Endometrium-Derived Mesenchymal Stem Cells and Their Tropism to Endometriosis. Stem Cells Int. 2017;2017:4794827. doi: 10.1155/2017/4794827. Epub 2017 Jul 6. PMID: 28761446; PMCID: PMC5518492.