Disease State
Etiology
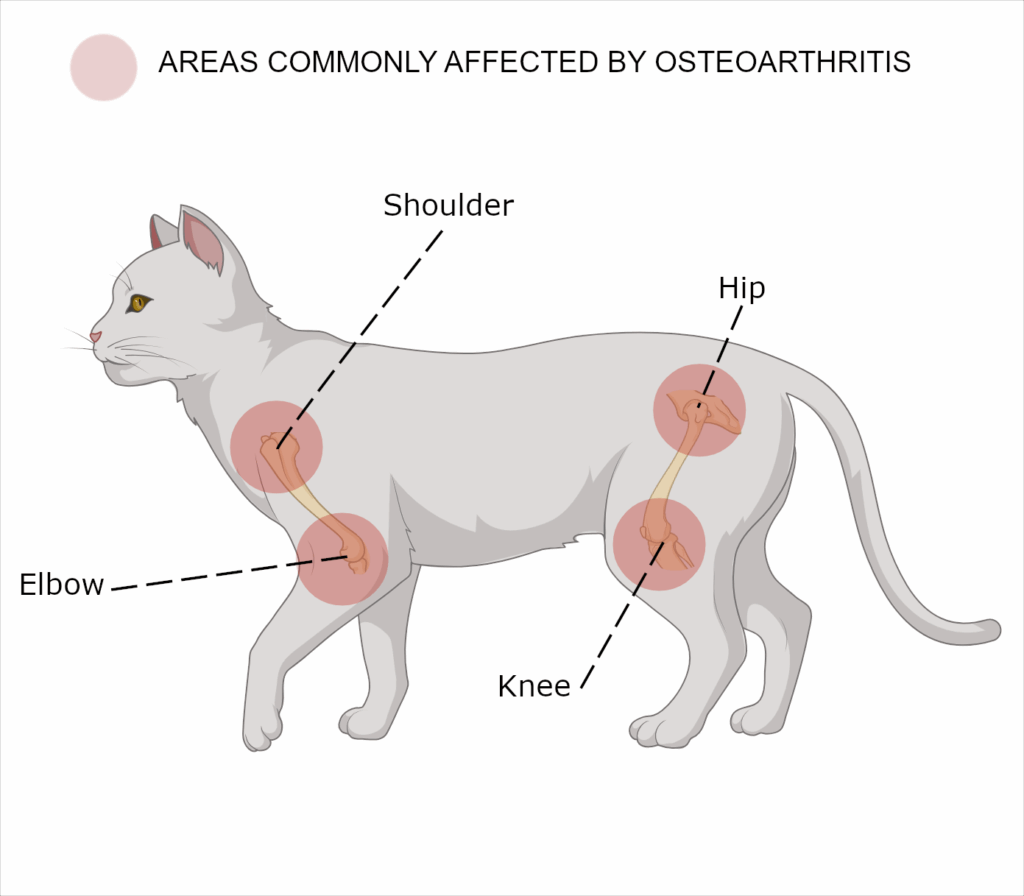
The reported prevalence of feline osteoarthritis (OA) varies widely (ranging from 22% to 93%), however, 80–90% of geriatric cats exhibit clinical or radiographic signs consistent with OA.1 In cats over six years of age, more than 60% are affected in at least one joint, and approximately 50% have OA involving multiple joints.1
OA in cats is predominantly primary or idiopathic in origin.2–4 Repetitive low-grade trauma is considered a potential contributor to OA pathogenesis in cats.5 Certain breeds, such as the Maine Coon, demonstrate increased susceptibility due to a genetic predisposition to hip OA.6,7 Additionally, some evidence suggests a possible increased prevalence in female cats.3,8 Unlike in dogs, the association between obesity and OA in cats is not well established.9–11
Clinical Signs
Recognizing osteoarthritis (OA) in cats can be more challenging than in dogs due to the subtle and often nonspecific nature of clinical signs. Cats experiencing pain and reduced mobility due to OA can have adverse secondary affects on their physical appearance, activity levels, mood, sociability, and overall physical and psychological well-being. This presentation combined with other more specific clinical signs (i.e. mobility changes) can help lead to an OA diagnosis.
Mobility-related changes may include:
- Decreased walking speed
- Reluctance or inability to jump
- Reduced jump height (e.g., failure to access elevated surfaces such as countertops)
- Hesitation or difficulty navigating stairs
Despite these changes, lameness is reported by owners in only 2-13% of OA-affected cats.8,9
Cats with OA may also exhibit inappropriate elimination behaviors, particularly if joint pain hinders their ability to enter or exit the litter box.9 Behavioral changes including increased irritability, withdrawal from social interactions, and aggression or aversion to handling are commonly observed and may serve as important clinical indicators of chronic pain.5,9,12
Owner burden in managing chronic OA
The burden of chronic medication administration for cats with OA can be daunting, leading to alterations in the caregiver/cat bond and inconsistent dosing. The caregiver burden for cats surpasses that seen in other species, and the burden of caring for cats with OA is reportedly higher than that seen for a variety of other diseases including cancer, heart disease, and diabetes.21 Stiff gait and jumping may improve with medical management but vocalization, resentment of handling, and/or aggression with handling often do not improve with symptomatic management.4
Diagnosis
Clinical Examination and Pain Assessment in Cats with Suspected Osteoarthritis
Conducting an orthopedic examination in cats can be particularly challenging and requires a low-stress, feline-friendly environment to improve accuracy and minimize behavioral confounders. It is possible to mistake fear-based behaviors for pain responses in a clinical setting.
General Appearance and Behavior
Postural and activity changes suggestive of discomfort or pain may include:
- Hunched or crouched posture
- Dorsolateral recumbency
- Abdominal muscle tension
- Vocalization
- Dull mentation or decreased responsiveness
- Restlessness or agitation
Behavioral alterations and environmental interaction cues often include:
- Withdrawal or reduced interest in surroundings
- Facing the back of the cage
- Reluctance to move after stimulation
- Attempting to hide or escape
- Irritability or aggression
- Changes in grooming behavior (either excessive or markedly reduced)
Facial expressions associated with discomfort may consist of:
- Ears pulled laterally or flattened
- Partially closed (squinting) eyes
- Tense muzzle
- Straightened whiskers
- Lowered head posture
Gait and Joint Evaluation
Gait evaluation in cats is notoriously difficult, as lameness is uncommonly reported and visual assessment in a clinical setting is often unreliable. When feasible, videos captured by owners of the cat ambulating in their home environment can provide valuable supplementary information.
Joint-specific examination involves palpation of individual joints, typically beginning with those suspected to be least painful and progressing to the most affected. Unfortunately, in feline patients, indicators such as joint pain, crepitus, effusion, thickening and decreased ROM are inconsistent and not as helpful for OA diagnosis as they are in other species.
Goniometric measurements of joint ROM can aid in identifying joints less likely to be affected by OA. In general, higher ROM values in joints that are non-painful on examination correlate with radiographically normal joints, making goniometry a potentially useful screening tool.16
Adjunctive Pain Monitoring Tools
Several objective tools are emerging to assess chronic pain in cats with OA, including:
- Collar-mounted accelerometers, which detect changes in activity patterns in response to analgesic treatment
- Piezoelectric activity monitors, which convert force, pressure, acceleration, and vibration into electrical signals to track movement patterns over time
Thermal and sensory threshold testing, which may help assess altered nociception in affected individuals
Radiographic imaging
Radiographic evidence suggests that approximately 91% of cats have osteoarthritis (OA) in at least one appendicular joint, with the shoulders, elbows, hips, stifles, and tarsi being the most frequently affected sites.1 Additionally, vertebral column OA is observed in 40–55% of cats, most commonly involving the lumbosacral joint. 1,8
The radiographic manifestations of OA in cats are unique compared to other species. In cats, the most common findings include periarticular new bone formation and joint-associated mineralization. Features such as joint effusion, osteophyte formation, and subchondral bone changes (commonly observed in canine OA) are less frequently detected in cats.17
Radiographic presentation of hip dysplasia also differs between species. In cats, the acetabulum often appears shallow, with remodeling and proliferative changes primarily affecting the cranio-dorsal acetabular margin, while remodeling of the femoral neck is typically minimal. Degenerative changes of the femoral head and neck develop slowly.18
Radiographs are not a reliable diagnostic for OA in cats as there is poor correlation between radiographic abnormalities and both clinical signs and cartilage damage in cats. Certain joints demonstrate a high prevalence of cartilage lesions even in the absence of radiographic evidence of disease including17:
- 71% of stifles
- 57% of coxofemoral joints
- 57% of elbows
- 46% of tarsal joints
There does appear to be a linear correlation between bone mineral density and degree of OA in the cat.18
Mechanism of Action
Pathophysiology of OA
In feline OA, cytokine profiling has revealed increased concentrations of inflammatory mediators, including tumor necrosis factor-alpha (TNF-α) and interleukin-2 (IL-2).13 Genomic studies have identified dysregulated immunomodulatory pathways related to antigen presentation (via MHC molecules), T-cell receptor signaling, and oxidative phosphorylation in cats with OA.14 Additionally, proteomic analyses have identified numerous differentially expressed proteins associated with feline OA and OA-related pain. These proteins are implicated in pathways such as the acute phase response and complement system activation, reinforcing the role of inflammation in disease progression.14
Given the relative avascularity and therefore the lack of systemic regulation, the repair capacity of cartilage is very limited. Over time, fibrous tissue forms with different functional properties then that of native hyaline cartilage, promoting joint degeneration.15
Current medical therapies
A multimodal approach is typically required to manage OA which often includes various combinations of non-steroidal anti-inflammatories (i.e meloxicam/ Metacam®, robenacoxib/ Onsior®), monoclonal Ab (frunevetmab/ Solensia®), pain medications (gabapentin/pregabalin, tramadol, buprenorphine, butorphanol, amantadine), and joint supplements (omega-3 fatty acids, chrondroitin-sulfate, other nutraceuticals). There are a small number of studies exploring the use of platelet-rich plasma (PRP) in cats.19 Adjunctive therapies, including physiotherapy and acupuncture, are also used in some cats. Most of these therapies typically work by blocking pain and inflammatory pathways to improve symptoms, as none have been shown to improve cartilage damage.20 Unfortunately this typically results in underlying disease progression even if the cats feel less painful.
Investigational Use of Mesenchymal Stem Cell Therapy in Feline Osteoarthritis
Mesenchymal stem cell (MSC) therapy is an emerging therapeutic approach currently under investigation for the treatment of osteoarthritis (OA) in cats. A pilot study evaluating allogeneic (donor is the same species as the recipient) adipose-derived MSCs in a small cohort of feline OA patients demonstrated increased activity levels in all treated cats, with two cats able to discontinue adjunctive medications and supplements.22 Importantly, no short-term or long-term adverse effects were reported.
The therapeutic effects of MSCs are believed to be mediated primarily through paracrine mechanisms, involving the secretion of bioactive molecules that modulate the local microenvironment. Studies in other species, including dogs and humans, have shown that MSC administration can suppress pro-inflammatory mediators, promote local tissue repair, and facilitate cartilage regeneration. Additionally, upregulation of genetic markers associated with chondrogenesis has been identified following MSC administration in canine models, supporting their potential to induce cartilage repair at a molecular level.
Unlike conventional OA therapies, MSCs offer a potential disease-modifying approach through their ability to restore joint homeostasis and stimulate cartilage regeneration. This unique regenerative mechanism distinguishes stem cell therapy from standard symptomatic treatments and positions it as a candidate for long-term management of feline OA.
Uterine derived MSCs
The endometrium is the lining of the uterus, and it undergoes cyclic regeneration during each menstrual cycle. As a result, it is a readily accessible and renewable source of stem cells. Obtaining allogeneic uterine-derived MSCs is less invasive and less complicated than other sources, making this cell source more feasible for therapeutic applications. Uterine-derived MSCs have also been found to possess potent immunomodulatory properties, including regulation of the immune response and reduction in inflammation, crucial for treating chronic inflammatory diseases.
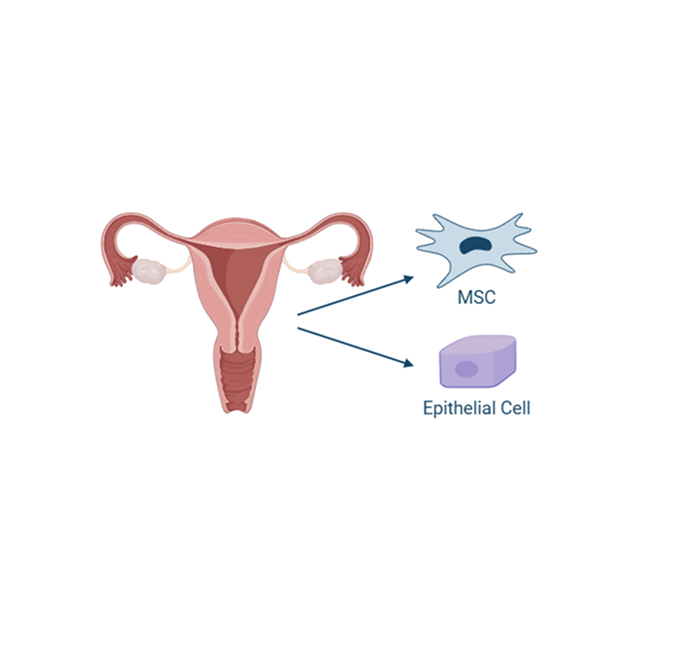
Additionally, MSCs derived from the uterus tend to have low expression of major histocompatibility complex (MHC) antigens, which makes them less likely to be recognized and attacked by the recipient’s immune system.
Available data to date suggests that use of MSCs could provide a novel treatment option for cats with OA, going beyond treatment of individual clinical signs to target the source of the disease, potentially enhancing their quality of life.
A deeper look into the life organ
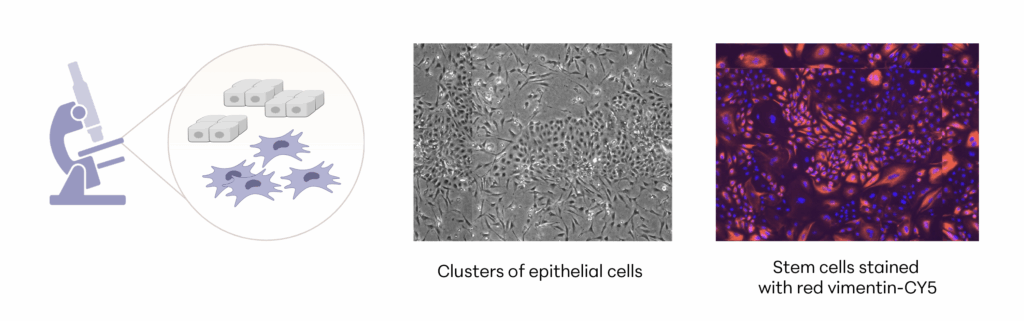
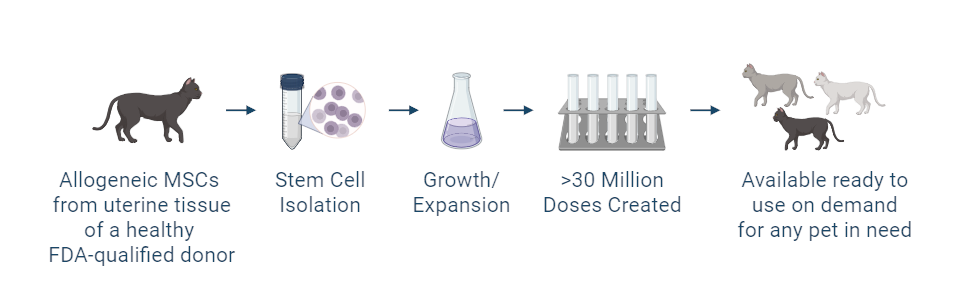
Manufacturing and Potency
The investigational stem cells being studied in pivotal studies are collected from an FDA-qualified feline donor during a routine spay procedure. The cells are intended to be used in other cats (same species) as an allogeneic ready to use product. The uterine-derived MSCs have been demonstrated in a matrix of assays to be potent (functional) and directed at the relevant clinical factors in feline OA. Each batch of stem cells is manufactured according to Current Good Manufacturing Practices (CGMP) and is released with established specifications demonstrating key quality attributes for identity, purity, safety and potency of the drug product.
Allogeneic cells from a FDA qualified donor
References
- Lascelles BDX, Henry Iii JB, Brown J, et al. Cross-Sectional Study of the Prevalence of Radiographic Degenerative Joint Disease in Domesticated Cats: Degenerative Joint Disease in Domestic Cats. Vet Surg. 2010;39(5):535-544. doi:10.1111/j.1532-950X.2010.00708.x
- Deabold K, Montalbano C, Miscioscia E. Feline Osteoarthritis Management. Vet Clin North Am Small Anim Pract. 2023;53(4):879-896. doi:10.1016/j.cvsm.2023.02.015
- Godfrey DR. Osteoarthritis in cats: a retrospective radiological study. J Small Anim Pract. 2005;46(9):425-429. doi:10.1111/j.1748-5827.2005.tb00340.x
- Clarke SP, Bennett D. Feline osteoarthritis: a prospective study of 28 cases. J Small Anim Pract. 2006;47(8):439-445. doi:10.1111/j.1748-5827.2006.00143.x
- Bennett D, Zainal Ariffin SMB, Johnston P. Osteoarthritis in the cat: 1. How common is it and how easy to recognise? J Feline Med Surg. 2012;14(1):65-75. doi:10.1177/1098612X11432828
- Low M, Eksell P, Högström K, Olsson U, Audell L, Ohlsson Å. Demography, heritability and genetic correlation of feline hip dysplasia and response to selection in a health screening programme. Sci Rep. 2019;9(1):17164. doi:10.1038/s41598-019-53904-w
- Loder RT, Todhunter RJ. Demographics of hip dysplasia in the Maine Coon cat. J Feline Med Surg. 2018;20(4):302-307. doi:10.1177/1098612X17705554
- Kimura T, Kimura S, Okada J, Suzuki S, Kitanaka T. Retrospective Radiographic Study of Degenerative Joint Disease in Cats: Prevalence Based on Orthogonal Radiographs. Front Vet Sci. 2020;7:138. doi:10.3389/fvets.2020.00138
- Slingerland LI, Hazewinkel HAW, Meij BP, Picavet Ph, Voorhout G. Cross-sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet J. 2011;187(3):304-309. doi:10.1016/j.tvjl.2009.12.014
- Clarke SP, Mellor D, Clements DN, et al. Prevalence of radiographic signs of degenerative joint disease in a hospital population of cats. Vet Rec. 2005;157(25):793-799. doi:10.1136/vr.157.25.793
- Bonecka J, Skibniewski M, Zep P, Domino M. Knee Joint Osteoarthritis in Overweight Cats: The Clinical and Radiographic Findings. Animals. 2023;13(15):2427. doi:10.3390/ani13152427
- Merola I, Mills DS. Behavioural Signs of Pain in Cats: An Expert Consensus. Suchodolski JS, ed. PLOS ONE. 2016;11(2):e0150040. doi:10.1371/journal.pone.0150040
- Gruen ME, Messenger KM, Thomson AE, et al. Evaluation of serum cytokines in cats with and without degenerative joint disease and associated pain. Vet Immunol Immunopathol. 2017;183:49-59. doi:10.1016/j.vetimm.2016.12.007
- Gao X, Lee J, Malladi S, Melendez L, Lascelles BDX, Al-Murrani S. Feline degenerative joint disease: a genomic and proteomic approach. J Feline Med Surg. 2013;15(6):466-477. doi:10.1177/1098612X12470652
- Dias I, Cardoso D, Soares C, et al. Clinical application of mesenchymal stem cells therapy in musculoskeletal injuries in dogs – A review of the scientific literature. Open Vet J. 2021;11(2):188. doi:10.5455/OVJ.2021.v11.i2.2
- Lascelles BDX, Dong YH, Marcellin-Little DJ, Thomson A, Wheeler S, Correa M. Relationship of orthopedic examination, goniometric measurements, and radiographic signs of degenerative joint disease in cats. BMC Vet Res. 2012;8(1):10. doi:10.1186/1746-6148-8-10
- Freire M, Robertson I, Bondell HD, et al. RADIOGRAPHIC EVALUATION OF FELINE APPENDICULAR DEGENERATIVE JOINT DISEASE VS. MACROSCOPIC APPEARANCE OF ARTICULAR CARTILAGE. Vet Radiol Ultrasound. 2011;52(3):239-247. doi:10.1111/j.1740-8261.2011.01803.x
- Yeowell G, Burns D, Fatoye F, et al. Indicators of Health-Related Quality of Life in Cats With Degenerative Joint Disease: Systematic Review and Proposal of a Conceptual Framework. Front Vet Sci. 2021;8:582148. doi:10.3389/fvets.2021.582148
- Huntingford J, Looney A, Johnson J, Miller L. The use of platelet rich plasma in the treatment of degenerative joint disease in cats: an exploratory case series. Front Vet Sci. 2024;11:1394055. doi:10.3389/fvets.2024.1394055
- Lefort-Holguin M, Delsart A, Frézier M, et al. Osteoarthritis in cats: what we know, and mostly, what we don’t know. . . yet. J Feline Med Surg. 2025;27(7):1098612X251347999. doi:10.1177/1098612X251347999
- Spitznagel MB, Gober MW, Patrick K. Caregiver burden in cat owners: a cross-sectional observational study. J Feline Med Surg. 2023;25(1):1098612X221145835. doi:10.1177/1098612X221145835