Stem Cell Therapy for Feline Chronic Kidney Disease (CKD): Investigating a New Way to Support Kidney Function in Cats
A pet parent’s guide to feline chronic kidney disease and how stem cell therapy is being studied as a potential new way to support kidney health and function.
Understanding Chronic Kidney Disease in Cats
Chronic kidney disease (CKD) is a progressive and irreversible condition that affects the kidneys’ ability to filter waste products from the blood. It is one of the most common medical issues seen in older cats, impacting an estimated 30–40% of cats over age 10.
When the kidneys are damaged, toxins build up in the bloodstream, leading to dehydration, loss of appetite, and weight loss. Over time, CKD can significantly affect a cat’s comfort and quality of life.
Recent research suggests that underlying inflammation may contribute to the onset and progression of kidney disease.
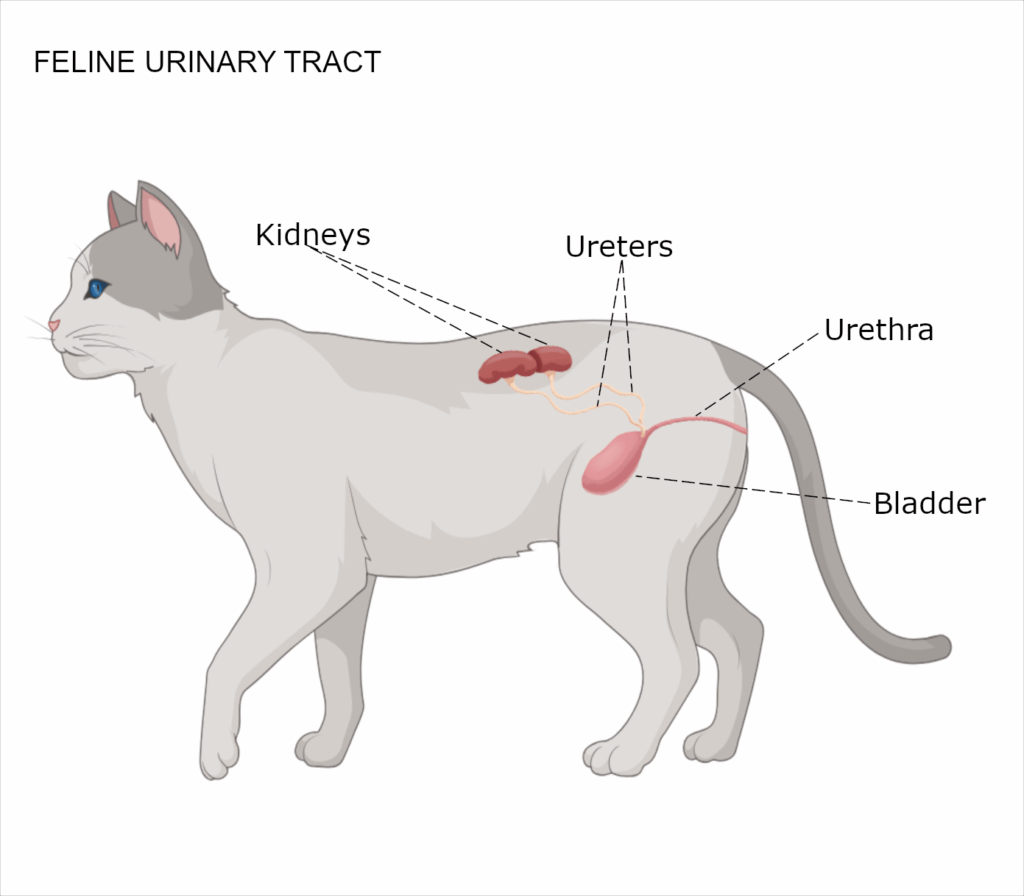
Common signs include:
- Increased thirst and urination
- Weight loss and decreased appetite
- Vomiting or nausea
- Lethargy or weakness
- Poor coat quality
Because CKD progresses slowly, early detection through routine bloodwork and urinalysis is essential.
Why Current Treatments Have Limitations
Conventional management of CKD focuses on slowing disease progression and maintaining hydration and nutrition. Treatments often include special prescription diets, medications to control blood pressure and phosphorus levels, antinausea medications and appetite stimulants, and subcutaneous fluid therapy.
While these approaches can help extend life and comfort, they do not address the underlying loss of kidney function. That gap has led researchers to explore regenerative medicine as a potential new avenue for supporting renal health.
Investigating Stem Cell Therapy for Feline CKD
Stem cell therapy, a branch of regenerative medicine, is being studied for its potential to modulate inflammation and support tissue repair—both key considerations in chronic kidney disease.
Gallant is investigating the use of uterine-derived mesenchymal stromal (stem) cells (UMSCs) in cats with CKD. These donor-derived cells are collected ethically during routine spay procedures and expanded under FDA-regulated conditions for investigational use.
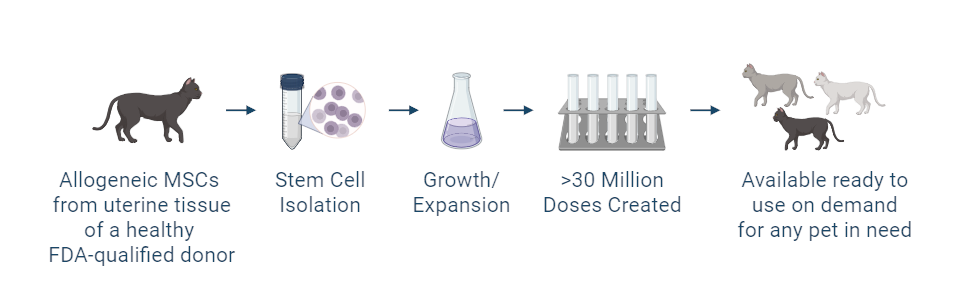
In early pilot studies, intravenous administration of allogeneic UMSCs was associated with improvements in selected clinical parameters in cats under investigation. While these early observations are encouraging, ongoing studies are needed to further evaluate safety, effectiveness, and potential clinical relevance.
How Gallant’s Research Works
Gallant’s investigational therapies are developed in a Current Good Manufacturing Practices (cGMP)–compliant facility to ensure consistency and quality. Each batch of cells is tested to verify cell identity, quality, and potency before use in clinical studies.
These FDA-authorized studies are designed to help researchers better understand whether stem cell therapy could one day become a safe, practical option to support veterinary care for chronic diseases like CKD.

Looking Ahead
CKD remains a major challenge for veterinarians and pet parents alike. Regenerative medicine represents a promising area of study that focuses on restoring balance rather than managing decline.
While stem cell therapy for feline CKD remains investigational, Gallant’s research is contributing to a growing understanding of how stem cells may help support kidney health and improve long-term well-being in cats.
Learn More
Gallant is leading the next generation of veterinary regenerative medicine, developing ready-to-use stem cell therapies designed to help pets live longer, healthier, and more comfortable lives.
Visit our Clinical Trials page
Learn more about participation and find a clinic near you.
All participation is voluntary and conducted under veterinary supervision in accordance with FDA regulations.
Disclaimer
Gallant’s investigational stem cell therapies are not commercially available. These veterinary products may be available through participation in a study at a qualified clinic under FDA-authorized protocols. This blog is intended for educational purposes only and does not constitute medical advice.


